Switzerland quarantine rule ‘could stop terminally ill accessing assisted death’








Switzerland quarantine rule ‘could stop terminally ill accessing assisted death’
Travel restrictions imposed on people returning from Switzerland could prevent terminally ill patients from having an assisted death, campaigners have said.Travel restrictions imposed on people returning from Switzerland could prevent terminally ill patients from having an assisted death, campaigners have said.
Travellers arriving in England after 4am on Saturday will need to self-isolate for 14 days, a rule which was also enforced in Scotland last week. Dignity in Dying said the rule change, announced late on Thursday for England, has made it “impossible to plan” for terminally ill patients hoping to travel to the Dignitas clinic in Switzerland.
It added that jobs and caring responsibilities, coupled with an average cost of £10,000 at Dignitas, could put the option of assisted dying out of reach for some people. Jane Parker was diagnosed with motor neurone disease in October 2019 and had been considering travelling to the clinic for an assisted death, which is illegal in the UK.
“I am now unable to speak and swallowing has become very difficult as the muscles continue to weaken,” the 68-year-old told the PA news agency.“I do not want to suffer a protracted, traumatic death but my options are severely limited, even more so since lockdown.

“Travelling to Switzerland for a legal assisted death, for example, is made even more difficult, particularly with this recently announced quarantine and travel restrictions changing practically every week.” This is how difficult planning an assisted death overseas was before the pandemic.
Ms Parker, who has three daughters and three grandchildren and lives with her husband Adrian in Devon, said: “I want to die at home, and am genuinely considering the idea of refusing food and water – a legal option under the current law.
“How can we allow people to starve and dehydrate to death, but not allow someone to slip away, quickly and peacefully in their sleep?” Dignity in Dying campaigns manager Ellie Ball told PA: “It’s just another source of anxiety about an option that is already so fraught with difficulty.
“For those who have the resources and strength to avail themselves of this choice, it takes many months of meticulous planning, often in secret. But with travel restrictions changing by the day, it makes planning almost impossible. “And for loved ones returning to the UK… imagine losing a dear relative, having to leave their body behind in a foreign land and then dealing with your grief completely alone with your support network?”
Ms Ball said the best solution is for assisted dying options to become available in the UK for “terminally ill, mentally competent people” in their final months.
In UK law, helping someone end their life is punishable by up to 14 years in prison.Before the coronavirus pandemic, Ms Ball said one Briton a week was travelling to Switzerland for an assisted death.
She added that each year around 300 terminally ill people end their own lives in the UK, often involving “multiple, incredibly traumatic attempts”.
The Government ruled out changing the law on assisted dying in 2019 after the high-profile case of Anne Whaley. Ms Whaley, then 76, was interviewed by police ahead of a planned journey with her 80-year-old husband Geoff Whaley, who had motor neurone disease, to Switzerland to end his life.
Before his death, Mr Whaley told the Times the stress of police involvement had “destroyed everything we had done to prepare ourselves”.
Reference: P A Media: By Edd Dracott, PA 2 days ago: 28/08/2020
Nevada man becomes first US known case of coronavirus reinfection










Nevada man becomes first US known case of coronavirus reinfection
Researchers have identified the first case of an American patient who was reinfected with the novel coronavirus. According to the report, the unnamed 25-year-old man, from Reno, Nevada, tested positive in April after showing mild illness.
He got sick again in late May and developed more severe COVID-19, the disease caused by the virus.Forty-eight days after his first positive test, he received a second one. 'This study likely represents a clear example of reinfection...reinfections are possible - which we already knew, because immunity is never 100 percent,' Dr Kristian Anderson, professor of immunology and microbiology at Scripps Research in La Jolla, California told Reuters.
Health experts are concerned that a future vaccine may not be very effective if reinfection is possible over such a short amount of time.During the first illness, the individual had symptoms including a sore throat, nausea, headache and diarrhea, according to the pre-print study.
He tested positive on April 18. His symptoms resolved by April 28 and he received two negative tests on May 9. Just a few weeks later, on May 31, the man reported symptoms including all the symptoms he had before as well as a fever and feeling dizzy. Five days later, the patient's condition worsened so much that he was hospitalized, and tested positive for SARS-CoV-2.
Bloodwork run by hospital staff showed that the man had antibodies against the virus. Researchers say the man was neither immunocompromised nor was he taking immunosuppressant drugs.A team at the University of Nevada, Reno School of Medicine and the Nevada State Public Health Laboratory said they were able to show through sophisticated testing that the virus associated with each instance of the Reno man's infection represented genetically different strains.

Because the genetic make-up of the strains are different enough, this represents a true reinfection. Researchers worked with the Washoe County Sheriff's Office Forensic Sciences Unit to determine that the samples were from the same patient.
They emphasized that reinfection with the virus is probably rare, but said the findingsimply that initial exposure to the virus may not result in full immunity for everyone.'We don't know at what frequency reinfections occur and how that might change over time,' Anderson said.
'Before we have broader studies illuminating these questions, we can't conclude what a single case of reinfection means for longevity and robustness of COVID-19 immunity and relevance for a future vaccine.' Mark Pandori, director of the Nevada State Public Health Laboratory, said: 'It is just one finding, but it shows that a person can possibly become infected with SARS-CoV-2 a second time.
'If reinfection is possible on such a short timeline, there may be implications for the efficacy of vaccines developed to fight the disease. It may also have implications for herd immunity.' Cases of presumed reinfection have cropped up in other parts of the world, but questions have arisen about testing accuracy.
Earlier this week, University of Hong Kong researchers reported details of a 33-year-old man who had recovered in April from a severe case of COVID-19 and was diagnosed four months later with a different strain of the virus.
Additionally, two European patients, one in Belgium and one in the Netherlands, were reported to have been reinfected with the virus. However, unlike the American man, the other patients developed mild cases of the virus during their second time with the infection.
'You'd expect the second time around people to have much milder or ideally no symptoms,' Dr Ashish Jha, director of the Harvard Global Health Institute, told NBC News.This is because the immune system should recognize the virus and be able to respond much more strongly than it did the first time around.
Jha says the case of the Hong Kong patient is 'completely consistent with that.' Pandori urged caution considering the many unknowns that still surround our knowledge about the immune responses to COVID-19. 'After one recovers from COVID-19, we still do not know how much immunity is built up, how long it may last, or how well antibodies play a role in protection against a reinfection,' he said in statement.
'If you've had it, you can't necessarily be considered invulnerable to the infection" a second time.
Mary Kekatos Senior Health Reporter For Dailymail.com and Reuters 1 day ago: 29/08/2020
Passenger caught coronavirus in plane toilet, says study











Passenger caught coronavirus in plane toilet, says study
A woman is believed to have caught coronavirus in the toilet of a plane according to a study published by the US Centers for Disease Control.A woman is believed to have caught coronavirus in the toilet of a plane according to a study published by the US Centers for Disease Control.
The study was based on evidence collected on an evacuation flight from Milan, Italy, to Incheon, South Korea, and at the government quarantine facility passengers were taken to after landing.
A total of 310 passengers were scheduled to the board the flight on 31 March, at the height of the pandemic.
It was “conducted under strict infection control procedures by the Korea Centers for Disease Control and Prevention (KCDC), based on the guidelines of the World Health Organization (WHO)” according to the research led by Dr. Sung Hwan Bae, a physician with the Soonchunhyang University College of Medicine in Seoul, South Korea.

Pre-boarding, passengers were examined by medical staff at the airport, which involved physical examinations, medical interviews and body temperature checks out of the airport.Eleven passengers who exhibited Covid-19 symptoms were removed from the flight, while 299 asymptomatic passengers were permitted to board the 11-hour flight to South Korea.
All passengers were given N95 respirators and kept 2m apart at the airport and on the plane.
“Most passengers wore the N95 respirators except at mealtimes and when using the toilet during the flight,” according to the study.Upon landing, all the passengers were taken directly to a government facility to quarantine in isolation and were tested for Covid on the 1st and 14th day of quarantine.
Six of the passengers tested positive for Covid on day one – they were transferred to hospital straight away but showed zero symptoms of coronavirus for the next 14 days and have since been classed as asymptomatic.The 28-year-old woman in question did not show symptoms when she boarded the flight in Italy, and had tested negative for Covid-19 when she began her 14-day quarantine.
But on the 14th day, tests came back positive.
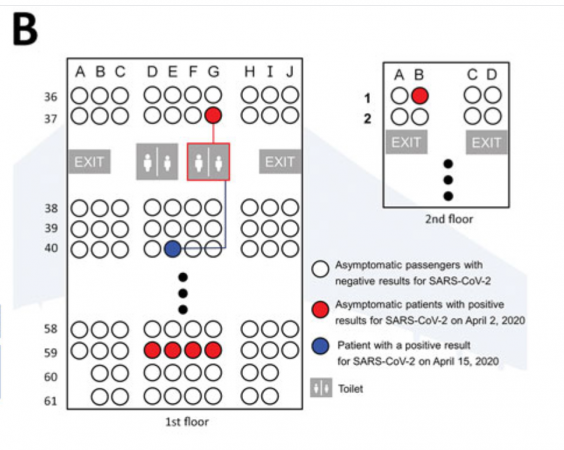
Given the conditions, researchers believed that she picked up Covid-19 after using the plane toilet that an asymptomatic carrier had used.The woman is said to have worn the N95 mask throughout the flight, except when she used the toilet.The study said: “The toilet was shared by passengers sitting nearby, including an asymptomatic patient. She was seated three rows away from the asymptomatic patient.
“Given that she did not go outside and had self-quarantined for three weeks alone at her home in Italy before the flight and did not use public transportation to get to the airport, it is highly likely that her infection was transmitted in the flight via indirect contact with an asymptomatic patient.”
The only alternative explanation, which researchers said was less likely, was that she had a longer than usual incubation period. In light of the findings, researchers recommended that to minimise transmission on aircraft, masks should be worn during the flight, hand hygiene should be maintained and social distancing measures should be maintained before boarding and after disembarkation.
Reference: Independent: Qin Xie 2 days ago: 27/08/2020
Unique clusters of type 2 diabetes identified in Indians










Unique clusters of type 2 diabetes identified in Indians
Researchers have identified distinct forms of type 2 diabetes unique to Asian Indians which could have implications for managing the disease.Researchers have identified distinct forms of type 2 diabetes unique to Asian Indians which could have implications for managing the disease.
Scientists said that Type 2 diabetes in Asian Indians, referred to as the “Asian Indian Phenotype”, differs from that in white Caucasians in a number of significant ways, however the majority of knowledge surrounding diabetes and its cardiovascular complications has been accumulated from studying white populations with Western European ancestry.
In the latest study, led by the University of Dundee’s Inspired project, researchers found that type 2 diabetes in Asian Indians can be classified into four distinct phenotypic clusters. While two of the clusters correspond to clusters identified in the European populations, the other two are novel sub-groups unique to Asian Indians.
The Asian Indian Phenotype is characterised by high levels of abdominal fat and increased insulin resistance even at low levels of body mass index (BMI), and it has been suggested this is the main reason for increased propensity to develop type 2 diabetes at younger age.

Researchers said classifying Asian Indians with type 2 diabetes into phenotypic clusters could help in predicting the risk of complications and in focusing more attention on individuals with the highest risk of developing these.
Professor Colin Palmer, of the University of Dundee’s School of Medicine, said: “These findings appear to be unique to Indians as they differ significantly from the findings published earlier in the European population.
“We recently reported that Asians respond better to DPP4 inhibitors and SGLT2 inhibitors. “The findings of this study confirm the greater insulin secretory defect and the younger age at onset of diabetes in South Asians.”
Inspired seeks to improve diabetes outcomes in India by working to better understand who gets diabetes, how it progresses, why some people respond better than others to treatments, and why some patients develop complications.
The university said the project sees Dundee’s expertise in the use of medical records to deliver improved care in diabetes “twinned” with the large patient data set (covering more than 450,000 Indian patients) collected by Dr Mohan’s Diabetes Specialities Centres, the largest clinical network of diabetes care in India.
The four clusters identified in the study are Severe Insulin Deficient Diabetes (SIDD), Insulin Resistant Obese Diabetes (IROD), Combined Insulin Resistant and Deficient Diabetes (CIRDD) and Mild Age-Related Diabetes (MARD).
SIDD and MARD correspond to the clusters identified in the Europeans populations, while the other two are novel sub-groups unique to the Asian Indian population.Researchers said CIRDD is of particular importance as it is
characterised by difficult-to-control hyperglycemia and increased risk of both diabetes eye and kidney disease.
Lead author Dr Viswanathan Mohan, chairman of Dr Mohan’s Diabetes Specialities Centre and President of Madras Diabetes Research Foundation, said: “These subgroups of type 2 diabetes have implications as far as treatment is concerned and the choice of anti-diabetic drugs.”
The study is published in BMJ Open Diabetes Research and Care. The research was funded by the National Institute for Health Research.
Reference: PA Media: By Lucinda Cameron, PA Scotland 2 days ago:26th August 2020
Articles - Most Read
- Home
- LIVER DIS-EASE AND GALL BLADDER DIS-EASE
- Contacts
- African Wholistics - Medicines, Machines and Ignorance
- African Wholistics -The Overlooked Revolution
- African Holistics - Seduced by Ignorance and Research
- The Children of the Sun-3
- Kidney Stones-African Holistic Health
- The Serpent and the RainBow-The Jaguar - 2
- PART ONE: DIS-EASE TREATMENT AND HEALTH-3
- 'Tortured' and shackled pupils freed from Nigerian Islamic school
- King Leopold's Ghost - Introduction
- PART ONE: DIS-EASE TREATMENT AND HEALTH-4
- PART ONE: DIS-EASE TREATMENT AND HEALTH-2
- PART ONE: DIS-EASE TREATMENT AND HEALTH-5
- African Wholistics - Medicine
- Menopause
- The Black Pharaohs Nubian Pharaohs of Ancient Egypt
- The Mystery System
- PART ONE: DIS-EASE TREATMENT AND HEALTH-6
Who's On Line?
We have 154 guests and no members online
Ad Agency Remote
Articles - Latest
- The Male G Spot Is Real—and It's the Secret to an Unbelievable Orgasm
- Herbs for Parasitic Infections
- Vaginal Care - From Pubes to Lubes: 8 Ways to Keep Your Vagina Happy
- 5 Negative Side Effects Of Anal Sex
- Your Herbs and Spices Might Contain Arsenic, Cadmium, and Lead
- Struggling COVID-19 Vaccines From AstraZeneca, BioNTech/Pfizer, Moderna Cut Incidence Of Arterial Thromboses That Cause Heart Attacks, Strokes, British Study Shows
- Cartilage comfort - Natural Solutions
- Stop Overthinking Now: 18 Ways to Control Your Mind Again
- Groundbreaking method profiles gene activity in the living brain
- Top 5 health benefits of quinoa
- Chromolaena odorata - Jackanna Bush
- Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins
- Doctors from Nigeria 'facing exploitation' in UK
- Amaranth, callaloo, bayam, chauli
- 9 Impressive Benefits of Horsetail
- Collagen The Age-Defying Secret Of The Stars + Popular Products in 2025
- Sarcopenia With Aging
- How to Travel as a Senior (20 Simple Tips)
- Everything you need to know about mangosteen
